
Attack reduction
Switch: attack reduction
Substantial and sustained reductions in HAE attack rates1-3
- Mean monthly HAE attack rate at Week 24 with DAWNZERA every 4 weeks was 0.44 vs 2.26 for placebo, an attack reduction of 81% (P<0.001, primary endpoint)1,2
- This increased to an 87% reduction when measured from the second dose (0.30 vs 2.25 for placebo, P<0.001, secondary endpoint)1,2
Reductions in mean monthly HAE attack rate (investigator-confirmed) from baseline3



Data cut off: January 27, 2025.3

REDUCTION
at 1 year in the open-label study
across both DAWNZERA dosing arms3†
Data cut off: January 27, 2025.3
Placebo-controlled study results:
- 53% of patients were attack free on DAWNZERA every 4 weeks (P=0.003 vs 9% with placebo)1,21†
- DAWNZERA every 8 weeks reduced HAE attacks by 55% vs placebo (P=0.004)1†

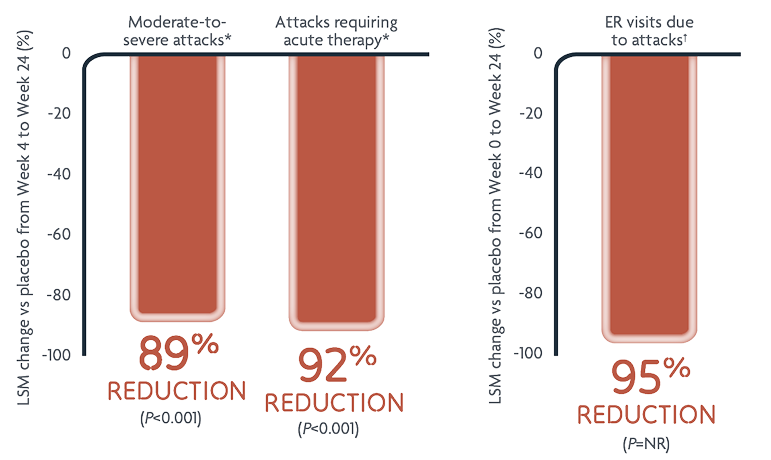
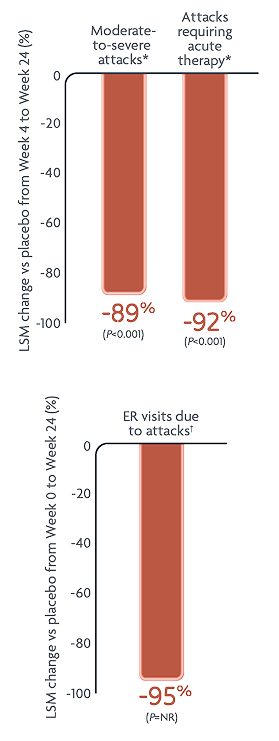
Reductions in HAE attack rates with DAWNZERA every 4 weeks (n=45)2

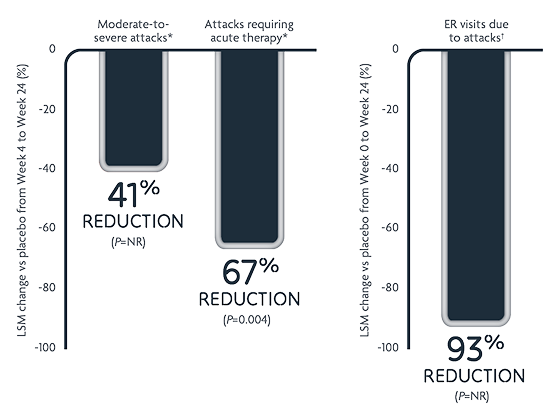
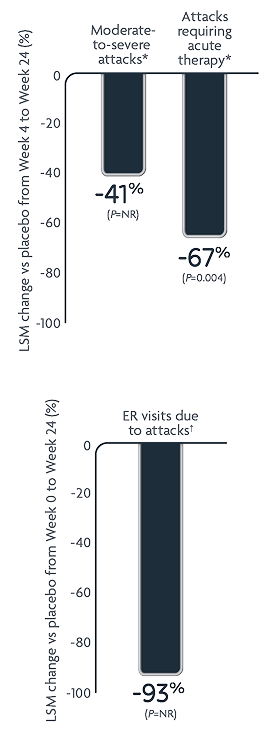
Reductions in HAE attack rates with DAWNZERA every 8 weeks (n=23)2.


Studied in patients starting HAE prophylactic therapy
Open-label extension cohort
(interim analysis at Week 52)
Open-label extension cohort
(interim analysis at Week 52)


