In the open-label study
Switching to DAWNZERA reduced mean HAE attack rate by 62% from prior treatment
Results in patients who switched to DAWNZERA every 4 weeks from an interim analysis at Week 161 *
Exploratory endpoint: reductions in mean monthly HAE attack rate with DAWNZERA1
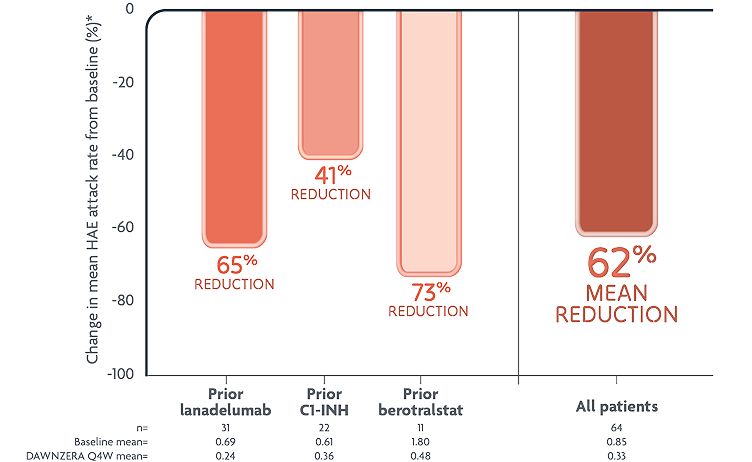
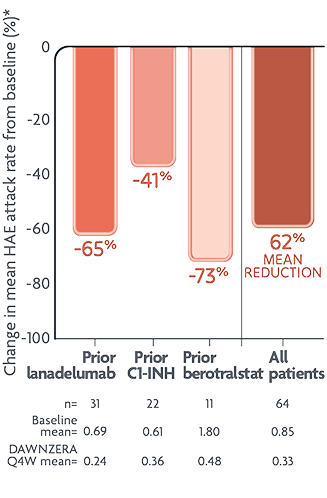
Baseline monthly HAE attack rate established in the 10-week screening period.1
- These are not head-to-head data. Findings are from an open-label, uncontrolled safety study in patients who wanted to switch to DAWNZERA, and was not powered for any comparisons between prior treatment groups; thus, these observations cannot be generalized to other patients on prior long-term prophylactic treatments
- The majority of adverse events were mild to moderate and consistent with the placebo-controlled study1,2


In the open-label study
Majority of patients who switched reported they preferred DAWNZERA
Patients successfully transitioned to DAWNZERA every 4 weeks from lanadelumab, berotralstat, or a C1-INH with no mean loss of efficacy and no new safety signals1
Exploratory endpoint: patient preference in an interim analysis at Week 16 (n=55)1

84% of patients surveyed who switched preferred DAWNZERA, most saying it worked better to control their HAE1
Reasons patients chose for preferring DAWNZERA*:
- 63% chose “it works better to control my HAE”
- 65% chose “less time to administer”
- 50% chose “less injection-site pain or reaction”
- These are not head-to-head data. Findings are from an open-label, uncontrolled safety study in patients who wanted to switch to DAWNZERA, and was not powered for any comparisons between prior treatment groups; thus, these observations cannot be generalized to other patients on prior long-term prophylactic treatments.
More than 1 reason was permitted.1

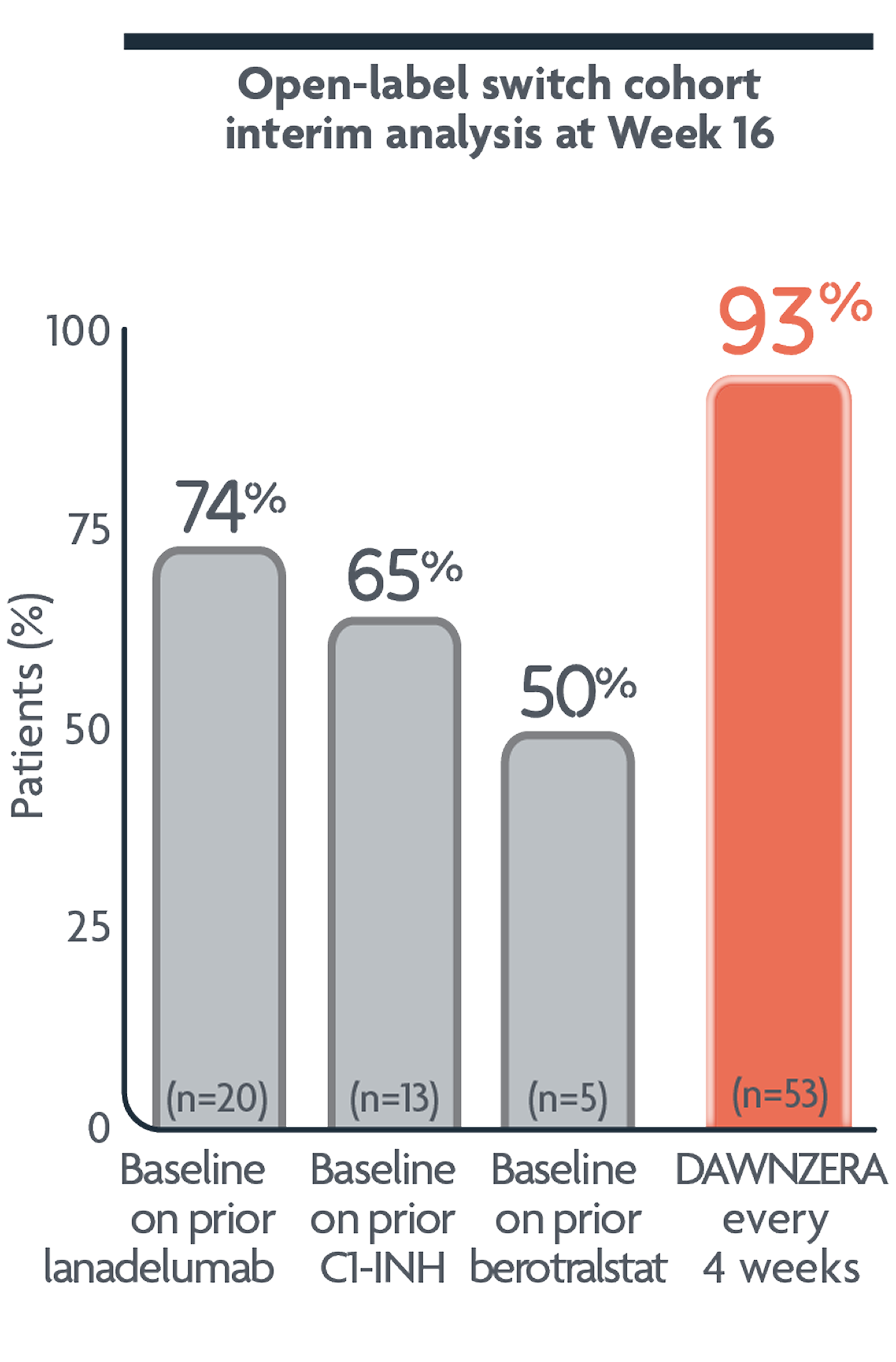
In the open-label study
93% of patients reported they were well-controlled with DAWNZERA1
- Disease control was self-reported by patients using the Angioedema Control Test (AECT)1
Well-controlled was defined as ≥10 out of 16 possible points on the AECT3*
Scores of 0 to 9 equated to poorly-controlled angioedema
Learn more about the AECT*The AECT was validated in 81 patients with recurrent angioedema, including 25 with HAE or acquired angioedema due to C1-INH deficiency. It was not specifically validated for HAE.3
These are not head-to-head data. Findings are from an open-label, uncontrolled safety study in patients who wanted to switch to DAWNZERA, and was not powered for any comparisons between prior treatment groups; thus, these observations cannot be generalized to other patients on prior long-term prophylactic treatments.
View switch protocol
Studied in patients switching to DAWNZERA
OASISplus switch cohort: Ongoing, open-label, long-term safety (primary) and efficacy (secondary) study of patients with HAE type 1 or 21
Prior therapies:
lanadelumab (n=31),
berotralstat (n=11),
or C1-INH (n=22)
Open-label switch cohort
(interim analysis at Week 16)
DAWNZERA 80 mg every 4 weeks (n=64)
- Enrolled patients on a stable dose of HAE prophylactic therapy for ≥12 weeks1
- Patients started taking DAWNZERA every 4 weeks on Day 1, following the 14-day switch protocol1
- Efficacy measures at Week 16 interim analysis were exploratory endpoints1