RNA Technology

The first and only RNA-targeted treatment for HAE1,2

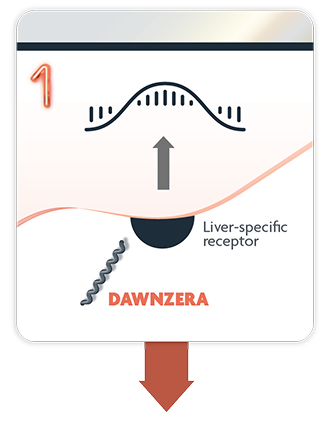
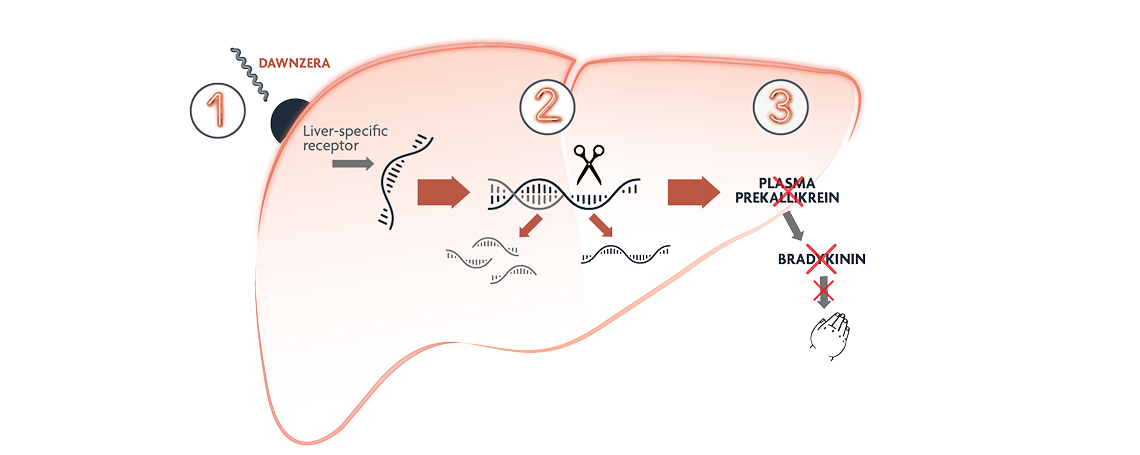
DAWNZERA is an antisense oligonucleotide (ASO) that binds to prekallikrein (PKK) mRNA to limit plasma protein production at the source—in the liver, where it’s made1,3

1. DAWNZERA is selectively targeted to the liver1,2,4
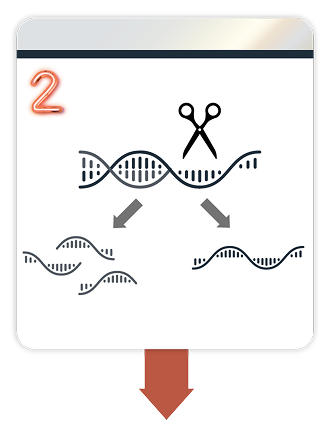
2. DAWNZERA binds to PKK mRNA and directs its degradation1,2,4
3. DAWNZERA reduces the production of PKK protein, reducing the release of bradykinin. This prevents the vascular leakage that characterizes HAE attacks1,2,4
DNA=deoxyribonucleic acid; mRNA=messenger ribonucleic acid.
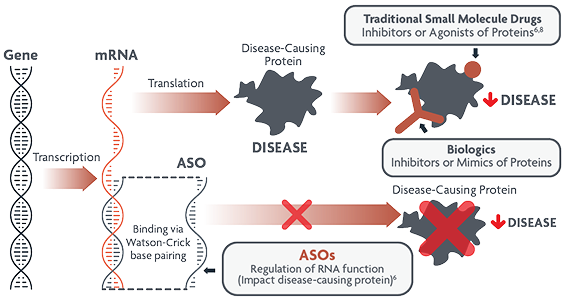
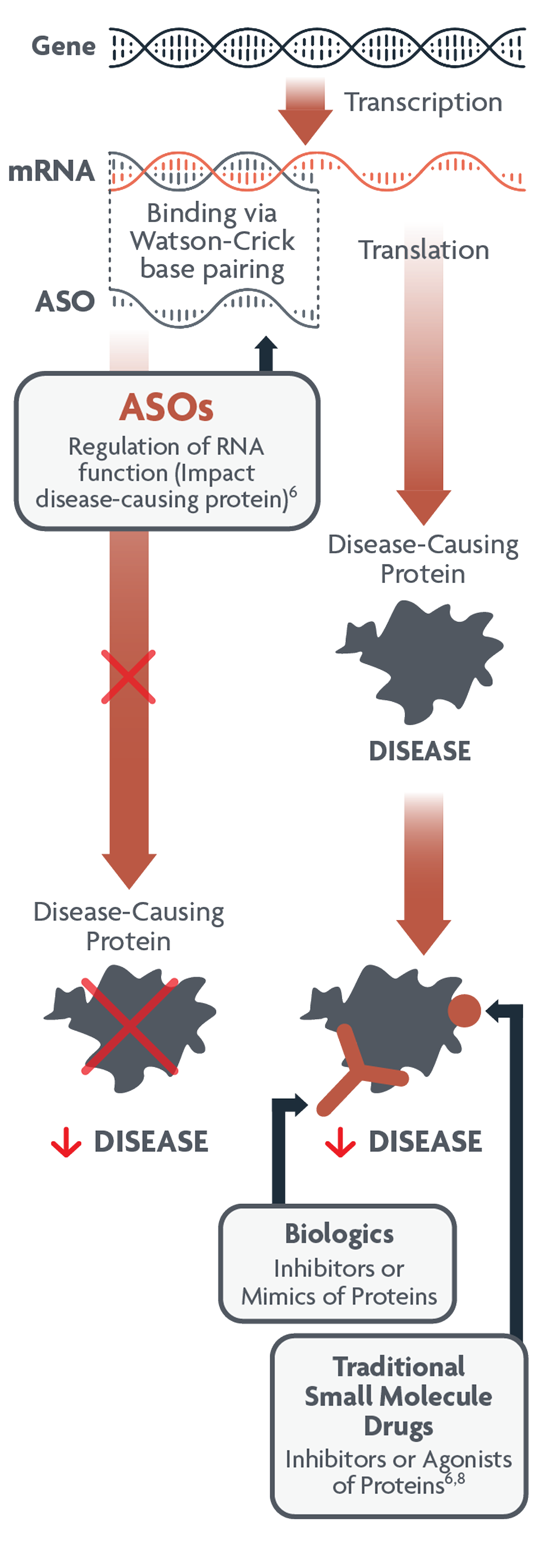
What is antisense technology?
Antisense oligonucleotides (ASOs) are a type of RNA-targeted therapy that can interfere with translation of specific proteins. They are designed to bind precisely with RNA and can either degrade or modify disease-causing proteins.6
-
•
Specifically target RNA6
-
•
Bind to complementary mRNA sequence7
-
•
Effects are reversible7
-
•
Leads to inhibiting gene expression or altering splicing7
-
•
Chemically modified to be stable: resist degradation by nucleases7
ASOs modulate gene expression and target different aspects of disease-causing proteins than small molecules and biologics6