
Quality of life
In the 6-month placebo-controlled study
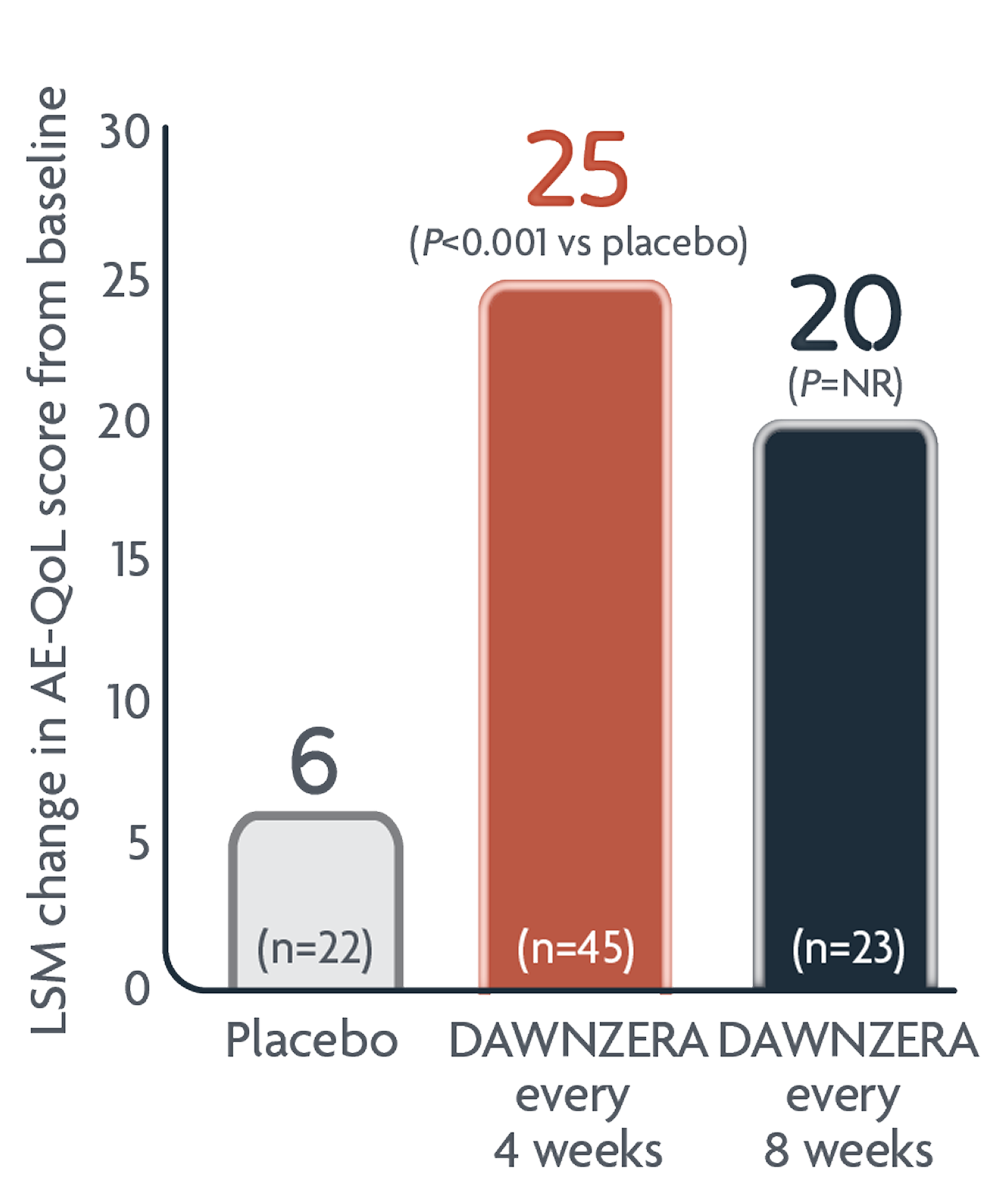
Patients reported improved QoL with DAWNZERA
DAWNZERA every 4 weeks significantly improved Angioedema Quality of Life (AE-QoL) scores—a validated measure in patients with angioedema1-3
Improvements from baseline in LSM AE-QoL score with DAWNZERA at Week 241

DAWNZERA
improved patient AE-QoL scores MORE THAN 3X beyond the minimum clinically important difference (>6 points)1




.svg)
Studied in patients switching to DAWNZERA
OASISplus switch cohort: Ongoing, open-label, long-term safety (primary) and efficacy (secondary) study of patients with HAE type 1 or 21
Prior therapies:
lanadelumab (n=31),
berotralstat (n=11),
or C1-INH (n=22)
Open-label switch cohort
(interim analysis at Week 16)
DAWNZERA 80 mg every 4 weeks (n=64)
- Enrolled patients on a stable dose of HAE prophylactic therapy for ≥12 weeks1
- Patients started taking DAWNZERA every 4 weeks on Day 1, following the 14-day switch protocol1
- Efficacy measures at Week 16 interim analysis were exploratory endpoints1
Studied in patients switching to DAWNZERA
OASISplus switch cohort: Ongoing, open-label, long-term safety (primary) and efficacy (secondary) study of patients with HAE type 1 or 21
Prior therapies:
lanadelumab (n=31),
berotralstat (n=11),
or C1-INH (n=22)
Open-label switch cohort
(interim analysis at Week 16)
DAWNZERA 80 mg every 4 weeks (n=64)
- Enrolled patients on a stable dose of HAE prophylactic therapy for ≥12 weeks1
- Patients started taking DAWNZERA every 4 weeks on Day 1, following the 14-day switch protocol1
- Efficacy measures at Week 16 interim analysis were exploratory endpoints1


